Topic 3.9. Sexual function in Friedreich ataxia
This chapter of the Clinical Management Guidelines for Friedreich Ataxia and the recommendations and best practice statements contained herein were endorsed by the authors and the Friedreich Ataxia Guidelines Panel in 2022.
Topic Contents
3.9.1 Disturbance of sexual function in Friedreich ataxia
3.9.2 Functional consequences of disturbance of sexual function
3.9.3 Assessment and management of sexual dysfunction
3.9.3.1 Assessment of sexual function
3.9.3.2 Management of sexual dysfunction
Disclaimer / Intended Use / Funding
Disclaimer
The Clinical Management Guidelines for Friedreich ataxia (‘Guidelines’) are protected by copyright owned by the authors who contributed to their development or said authors’ assignees.
These Guidelines are systematically developed evidence statements incorporating data from a comprehensive literature review of the most recent studies available (up to the Guidelines submission date) and reviewed according to the Grading of Recommendations, Assessment Development and Evaluations (GRADE) framework © The Grade Working Group.
Guidelines users must seek out the most recent information that might supersede the diagnostic and treatment recommendations contained within these Guidelines and consider local variations in clinical settings, funding and resources that may impact on the implementation of the recommendations set out in these Guidelines.
The authors of these Guidelines disclaim all liability for the accuracy or completeness of the Guidelines, and disclaim all warranties, express or implied to their incorrect use.
Intended Use
These Guidelines are made available as general information only and do not constitute medical advice. These Guidelines are intended to assist qualified healthcare professionals make informed treatment decisions about the care of individuals with Friedreich ataxia. They are not intended as a sole source of guidance in managing issues related to Friedreich ataxia. Rather, they are designed to assist clinicians by providing an evidence-based framework for decision-making.
These Guidelines are not intended to replace clinical judgment and other approaches to diagnosing and managing problems associated with Friedreich ataxia which may be appropriate in specific circumstances. Ultimately, healthcare professionals must make their own treatment decisions on a case-by-case basis, after consultation with their patients, using their clinical judgment, knowledge and expertise.
Guidelines users must not edit or modify the Guidelines in any way – including removing any branding, acknowledgement, authorship or copyright notice.
Funding
The authors of this document gratefully acknowledge the support of the Friedreich Ataxia Research Alliance (FARA). The views and opinions expressed in the Guidelines are solely those of the authors and do not necessarily reflect the official policy or position of FARA.
3.9 Sexual function in Friedreich ataxia
Louise Corben, Paola Giunti, Anton Emmanuel, Jalesh N. Panicker and Marios Hadjivassiliou
This chapter describes the effects of Friedreich ataxia on sexual function, the functional consequences of these effects, and strategies for assessing and managing sexual dysfunction. In making recommendations for management, the authors were tasked with answering the question:
For individuals with Friedreich ataxia, what management strategies could be implemented for disturbance of sexual function?
3.9.1 Disturbance of sexual function in Friedreich ataxia
Two studies indicate that people with Friedreich ataxia (FRDA) may experience difficulties in intimate sexual relationships (1, 2). In one study, sexual dysfunction was quantified in 83% (30/36) of individuals with FRDA administered the Arizona Sexual Experience Scale (2). Likewise, the impact of FRDA on sexual functioning, sexual satisfaction and the capacity to form intimate relationships was explored in an online survey completed by 107 individuals with FRDA (1). Erectile dysfunction was reported in 57% (20/35) of males, inadequate vaginal lubrication interfering with sexual responsiveness was reported in 57.7% (26/45) of females, and reduced genital sensation in 47% (51/107) of respondents. In addition, 88% (94/107) reported problems moving their body during sexual activity due to muscle spasm and/or tightness, fatigue and weakness, and 73% (78/107) reported reduced confidence about their sexuality due to FRDA (1).
3.9.2 Functional consequences of disturbance of sexual function
The presence of FRDA may impact sexual functioning, sexual satisfaction and the capacity to form intimate relationships. The sexual response is a complex phenomenon (3), and in people with FRDA may well be influenced by multiple factors including the function of neurological, cardiac or vascular systems; attitudes towards sexuality; or the individual’s self-esteem or self-worth (4). In addition, individuals report that the psychological, social and emotional effects of living with FRDA does affect sexual activity and fulfilment (1). Sexual dysfunction has not been shown to be related to clinical severity (2); however, a relationship between a younger age of onset and sexual dysfunction has been reported, reinforcing the importance of asking questions about sexual function as soon as appropriate (1).
3.9.3 Assessment and management of sexual dysfunction
3.9.3.1 Assessment of sexual function
Sexual dysfunction in people with neurological conditions can be devastating (4). It is essential the clinician does not ignore this fundamental aspect of a person’s life and individuals with FRDA should be encouraged to discuss sexual function in the clinical setting (1). In particular, it is important that the clinician establishes whether sexual dysfunction is a problem and clarifies the nature, history and characteristics of any sexual dysfunction. This should include details of libido, genital sensation, erectile function, lubrication, ejaculation, orgasm and menstrual function. A clinical examination and investigations may also be indicated as shown in Figure 3.9.1. The effect of concurrent conditions such as depression, and medications such as SSRIs and anti-hypertensives should also be explored (5).
3.9.3.2 Management of sexual dysfunction
A careful history will identify if primary aspects of sexual dysfunction are amenable to pharmacological intervention (such as phosphodiesterase-5 inhibitors in the case of erectile dysfunction) or if interventions to manage associated issues, such as depression or physical or sensory impairment compromising sexual function, are indicated. Whilst there is currently no direct evidence related to FRDA to support referral to psychologists and/or sex therapists for counseling, there may be benefit from advice regarding alternative sexual positions, use of stimulation devices and techniques, managing fatigue and managing reduced self-esteem (4, 6). The role of counseling in managing sexual dysfunction warrants further empirical attention.
Figure 3.9.1 Algorithm summarizing management approaches (from Corben et al, 2021 (1))
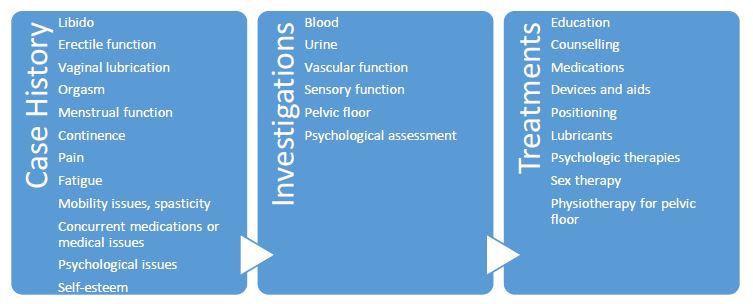
There is little evidence regarding the benefits of commencing a phosphodiesterase-5 inhibitor in males with FRDA who report erectile dysfunction; however, there is sufficient evidence to indicate erectile dysfunction may be a problem and evidence in other neurological conditions that treating erectile dysfunction with phosphodiesterase-5 inhibitors would be of benefit (7). Treatment goals should be balanced between the needs of the person and potential side effects, including possible cardiac impacts.
It is not clear if low testosterone is a consistent feature of FRDA, so routine testing of serum testosterone in men reporting sexual dysfunction is not supported. However, we suggest morning serum total testosterone should be tested in males with FRDA with clinically suspected hypogonadism, or if first-line treatment of erectile dysfunction is unsuccessful.
Whilst high rates of poor vaginal lubrication are documented in women with FRDA there is no evidence to support the use of estrogen creams in FRDA. Moreover, the use of these agents would depend upon a risk assessment, particularly in terms of the individual’s risk for developing breast or uterine cancer. Vaginal lubricants (aqueous/oil-based/silicone) are generally the first-line option for managing poor vaginal lubrication.
Enquiring about sexual function
QUESTION: Should enquiring about sexual function including erectile or vaginal lubrication dysfunction versus no enquiry be used for all sexually active people with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: ↑↑LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We recommend clinicians enquire about sexual function, including but not limited to erectile or vaginal lubrication dysfunction, the physical capacity to engage in sexual activity and the psychological aspect of the sexual response in sexually active individuals with Friedreich ataxia.
JUSTIFICATION: There are two recent published studies that indicate sexual dysfunction is a greater issue in individuals with Friedreich ataxia than those without (1, 2). It is important that the clinician enquires about the possibility to ensure appropriate intervention can be implemented aimed at improving intimate relationships.
SUBGROUP CONSIDERATION: This recommendation is for sexually active individuals with Friedreich ataxia.
Evidence to Recommendation Table PDFCounseling for sexual dysfunction
QUESTION: Should counseling for sexual dysfunction versus no counseling be used for all sexually active people who report sexual dysfunction with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: —LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We cannot recommend either counseling or no counseling to improve quality of life, self-esteem, management of physical or sensory impairment compromising sexual function, or intimate relationships in sexually active people with Friedreich ataxia reporting sexual dysfunction.
JUSTIFICATION: There is little evidence supporting the role for counseling in the management of Friedreich-ataxia-related sexual dysfunction, although there are studies in other cohorts that suggest a possible role.
SUBGROUP CONSIDERATION: This recommendation is for individuals with Friedreich ataxia who report sexual dysfunction.
Evidence to Recommendation Table PDFPhosphodiesterase-5 inhibitor for males with erectile dysfunction
QUESTION: Should commencing a phosphodiesterase-5 inhibitor versus no treatment be used for sexually active males with erectile dysfunction with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: ↑↑LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We recommend commencing a phosphodiesterase-5 inhibitor in males with Friedreich ataxia who report erectile dysfunction.
JUSTIFICATION: There is little evidence regarding the benefits of commencing a phosphodiesterase-5 inhibitor in males with Friedreich ataxia who report erectile dysfunction; however, there is sufficient evidence to indicate erectile dysfunction may be a problem and evidence in other neurological conditions that phosphodiesterase-5 inhibitor therapy can be of benefit in this setting.
SUBGROUP CONSIDERATION: This recommendation is for sexually active males with Friedreich ataxia who report erectile dysfunction and who are not taking nitrates for coexistent angina.
Evidence to Recommendation Table PDFNatural estrogen for inadequate vaginal lubrication
QUESTION: Should commencing a course of natural estrogen versus no treatment be used for sexually active females with inadequate vaginal lubrication with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: ↓↓LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We recommend not commencing a course of natural estrogen in sexually active females with inadequate vaginal lubrication with Friedreich ataxia.
JUSTIFICATION: While high rates of poor vaginal lubrication are documented in women with Friedreich ataxia, there is no evidence for use of estrogen creams in Friedreich ataxia. Moreover, the use of these agents would depend upon a risk assessment, particularly in terms of the individual’s risk for developing breast/uterine cancer. Vaginal lubricants (aqueous/oil-based/silicone) are generally the first-line option for managing poor vaginal lubrication.
SUBGROUP CONSIDERATION: This recommendation is for sexually active females with Friedreich ataxia with poor vaginal lubrication.
Evidence to Recommendation Table PDFMeasuring morning serum testosterone level
QUESTION: Should measuring testosterone levels versus no testing be used for all sexually active men reporting sexual dysfunction with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: —LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We cannot recommend either testing morning serum testosterone or not measuring morning serum testosterone in all sexually active men with Friedreich ataxia reporting sexual dysfunction; however, we suggest morning serum total testosterone should only be tested in males with Friedreich ataxia with clinically suspected hypogonadism, or if first-line treatment of erectile dysfunction is unsuccessful.
JUSTIFICATION: Two studies show that sexual dysfunction is prevalent in individuals with Friedreich ataxia (1, 2). From the available evidence, low testosterone levels do not appear to be a consistent feature in Friedreich ataxia and this has not been assessed in men with Friedreich ataxia reporting sexual dysfunction. Hence, routine assessment in every male with Friedreich ataxia reporting sexual dysfunction cannot be recommended.
SUBGROUP CONSIDERATION: This recommendation is for sexually active males reporting sexual dysfunction. Sexually active males with Friedreich ataxia with clinically suspected hypogonadism or those for whom first-line treatment of erectile dysfunction has not been successful could have morning serum total testosterone measured.
Evidence to Recommendation Table PDFLay summary of clinical recommendations for disturbance of sexual function in Friedreich ataxia
Why these recommendations?
Two published studies have indicated that people with Friedreich ataxia may experience difficulties in intimate sexual relationships, with problems such as achieving and maintaining an erection (erectile dysfunction), inadequate vaginal lubrication, or difficulties related to physical problems or low self-esteem. We therefore recommend that healthcare professionals should ask people with Friedreich ataxia whether they are having any of these problems.
In terms of treatment, there is currently little evidence that shows benefits from counseling in improving quality of life or self-esteem, or management of physical problems related to sexual function or intimate relationships in sexually active people with Friedreich ataxia, but more research is needed. However, there is some evidence that using a medication that helps erections may be useful for men with Friedreich ataxia who report problems with erections. Likewise, vaginal lubricants (water/oil/silicone-based) may be of benefit to women reporting poor vaginal lubrication.
What does this mean for you as a person living with Friedreich ataxia or caring for someone living with Friedreich ataxia?
It might be important for you to speak with your healthcare professional about Friedreich ataxia and sexual function, what it means for you, and if treatment would be useful in your particular circumstances.
Who are these recommendations specifically for?
This recommendation is specifically for men or women with Friedreich ataxia who are sexually active.
Principal Research Fellow, Murdoch Children’s Research Institute, Melbourne, Victoria, Australia
Email: louise.corben@mcri.edu.au
Anton Emmanuel, MD, FRCP
Consultant NeuroGastroenterologist, National Hospital Neurology & Neurosurgery, London, UK
Paola Giunti, MD, PhD
Professor, Queen Square Institute of Neurology, UCL, London, UK
Email: p.giunti@ucl.ac.uk
Marios Hadjivassiliou, MD
Professor of Neurology, Sheffield Teaching Hospitals NHS Trust and University of Sheffield, Sheffield, South Yorkshire, UK
Jalesh N. Panicker, MD, DM, FRCP
Associate Professor and Consultant Neurologist in Uro-Neurology, The National Hospital for Neurology and Neurosurgery and UCL Queen Square Institute of Neurology, London, UK
2. Lad M, Parkinson MH, Rai M, Pandolfo M, Bogdanova-Mihaylova P, Walsh RA, et al. Urinary, bowel and sexual symptoms in a cohort of patients with Friedreich’s ataxia. Orphanet J Rare Dis. 2017;12(1):158.
3. Nasimbera A, Rosales J, Silva B, Alonso R, Bohorquez N, Lepera S, et al. Everything you always wanted to know about sex and neurology: neurological disability and sexuality. Arq Neuropsiquiatr. 2018;76(7):430-5.
4. Matthews V. Sexual dysfunction in people with long-term neurological conditions. Nurs Stand. 2009;23(50):48-56; quiz 8.
5. Basson R, Bronner G. Management and rehabilitation of neurologic patients with sexual dysfunction. Handb Clin Neurol. 2015;130:415-34.
6. Lundberg PO, Ertekin C, Ghezzi A, Swash M, Vodusek D. Neurosexology: Guideline for neurologists. Eur J Neurol. 2001;8 (Suppl 3):2-24.
7. Lombardi G, Nelli F, Celso M, Mencarini M, Del Popolo G. Treating erectile dysfunction and central neurological diseases with oral phosphodiesterase type 5 inhibitors. Review of the literature. J Sex Med. 2012;9(4):970-85.
These Guidelines are systematically developed evidence statements incorporating data from a comprehensive literature review of the most recent studies available (up to the Guidelines submission date) and reviewed according to the Grading of Recommendations, Assessment Development and Evaluations (GRADE) framework © The Grade Working Group.
This chapter of the Clinical Management Guidelines for Friedreich Ataxia and the recommendations and best practice statements contained herein were endorsed by the authors and the Friedreich Ataxia Guidelines Panel in 2022.
It is our expectation that going forward individual topics can be updated in real-time in response to new evidence versus a re-evaluation and update of all topics simultaneously.

