Topic 3.8. Lower urinary tract and bowel function in Friedreich ataxia
This chapter of the Clinical Management Guidelines for Friedreich Ataxia and the recommendations and best practice statements contained herein were endorsed by the authors and the Friedreich Ataxia Guidelines Panel in 2022.
Topic Contents
3.8.1 Overview of lower urinary tract dysfunction in Friedreich ataxia
3.8.1.1 Prevalence of lower urinary tract symptoms
3.8.1.2 Pattern of lower urinary tract dysfunction in Friedreich ataxia
3.8.2 Management of lower urinary tract dysfunction
3.8.2.1 Literature review
3.8.2.2 Evaluation of lower urinary tract symptoms
3.8.2.3 Management of LUT symptoms
3.8.2.4 Management of recurrent urinary tract infection
3.8.3 Overview of bowel function in Friedreich ataxia
3.8.3.1 Prevalence
3.8.3.2 Effects of Friedreich ataxia on bowel function
3.8.4 Management of bowel dysfunction
Disclaimer / Intended Use / Funding
Disclaimer
The Clinical Management Guidelines for Friedreich ataxia (‘Guidelines’) are protected by copyright owned by the authors who contributed to their development or said authors’ assignees.
These Guidelines are systematically developed evidence statements incorporating data from a comprehensive literature review of the most recent studies available (up to the Guidelines submission date) and reviewed according to the Grading of Recommendations, Assessment Development and Evaluations (GRADE) framework © The Grade Working Group.
Guidelines users must seek out the most recent information that might supersede the diagnostic and treatment recommendations contained within these Guidelines and consider local variations in clinical settings, funding and resources that may impact on the implementation of the recommendations set out in these Guidelines.
The authors of these Guidelines disclaim all liability for the accuracy or completeness of the Guidelines, and disclaim all warranties, express or implied to their incorrect use.
Intended Use
These Guidelines are made available as general information only and do not constitute medical advice. These Guidelines are intended to assist qualified healthcare professionals make informed treatment decisions about the care of individuals with Friedreich ataxia. They are not intended as a sole source of guidance in managing issues related to Friedreich ataxia. Rather, they are designed to assist clinicians by providing an evidence-based framework for decision-making.
These Guidelines are not intended to replace clinical judgment and other approaches to diagnosing and managing problems associated with Friedreich ataxia which may be appropriate in specific circumstances. Ultimately, healthcare professionals must make their own treatment decisions on a case-by-case basis, after consultation with their patients, using their clinical judgment, knowledge and expertise.
Guidelines users must not edit or modify the Guidelines in any way – including removing any branding, acknowledgement, authorship or copyright notice.
Funding
The authors of this document gratefully acknowledge the support of the Friedreich Ataxia Research Alliance (FARA). The views and opinions expressed in the Guidelines are solely those of the authors and do not necessarily reflect the official policy or position of FARA.
3.8 Lower urinary tract and bowel function in Friedreich ataxia
Jalesh N. Panicker, Anton Emmanuel, Paola Giunti, David J. Szmulewicz and Marios Hadjivassiliou
This chapter describes the effects of Friedreich ataxia on lower urinary tract and bowel function, the prevalence and pattern of lower urinary tract and bowel dysfunction in people with Friedreich ataxia, and strategies for managing disturbance of lower urinary tract and bowel function. In making recommendations for management, the authors were tasked with answering the question:
For individuals with Friedreich ataxia, what management strategies could be implemented for disturbance of bladder and bowel function?
3.8.1 Overview of lower urinary tract dysfunction in Friedreich ataxia
3.8.1.1 Prevalence of lower urinary tract symptoms
From the limited data available, lower urinary tract (LUT) symptoms appear to be a common problem in people with Friedreich ataxia (FRDA), most commonly urinary storage symptoms (1, 2). Lad and colleagues demonstrated a high prevalence of 80% using validated questionnaires (1). Delatycki and colleagues (3) observed that 41% of a cohort of individuals with FRDA from eastern Australia with homozygous FXN intron 1 GAA expansions reported LUT symptoms, and this did not correlate with the size of the gene mutation. Durr and colleagues (4) noted a prevalence of 23% in their cohort of 140 people.
3.8.1.2 Pattern of lower urinary tract dysfunction in Friedreich ataxia
Information about the pattern of LUT dysfunction in individuals with FRDA is sparse. It has been established that symptoms tend to correlate with disease duration (3). Vezina and colleagues (5) observed that individuals with FRDA most often reported overactive bladder symptoms, with urgency incontinence being the commonest. The problem is made worse when mobility is affected and it is difficult to reach and transfer to the toilet in time. More recent data derived from the Collaborative Clinical Research Network (CCRN) in FRDA sheds light into the extent of LUT symptoms. Amongst 578 individuals included in this database, urgency was reported in 31% and incontinence in 27%. Ninety-one (16%) people reported frequent urinary incontinence (greater than once a week), amongst whom two required catheterization and 28% reported that bladder problems had altered their activities. However, only 13% were on medications for LUT symptoms (Cooperative Clinical Research Network in Friedreich Ataxia, J Farmer, D Lynch, and A Brocht, unpublished observations).
The overactive bladder is the most common presentation of LUT dysfunction in FRDA. This is a result of the detrusor muscle of the bladder contracting involuntarily and unpredictably. Urodynamic studies demonstrate different changes during the storage phase, including uninhibited contractions (detrusor overactivity) and reduced bladder capacity (2, 5, 6). These changes presumably reflect pyramidal tract involvement in FRDA (7). During the voiding phase of urodynamics, detrusor underactivity has been demonstrated (2). The commonest finding in needle electromyography (EMG) of the external urethral sphincter is complete voluntary relaxation (5). The risk of upper urinary tract damage is low, and though Musegante and colleagues reported that 14% of individuals were found to have dilatation of the upper urinary tract in ultrasonography, none had altered creatinine levels (2).
3.8.2 Management of lower urinary tract dysfunction
3.8.2.1 Literature review
An extensive literature search did not uncover any publications that specifically addressed the management of LUT symptoms in FRDA. Therefore, the guidelines proposed are based on limited understanding of the pathophysiology of LUT dysfunction in individuals with FRDA reported in a cross-sectional observational study (1) (level IV evidence). Urological issues in people with FRDA should be managed by a suitably trained healthcare professional who is knowledgeable about the ataxias and neurogenic LUT dysfunction. In most cases, LUT symptoms can be successfully managed using a bedside algorithm which has been adopted from treatment guidelines for managing LUT dysfunction in patients with multiple sclerosis (see Figure 3.8.1) (8).
3.8.2.2 Evaluation of lower urinary tract symptoms
The first step when evaluating LUT symptoms in people with FRDA is to test for a urinary tract infection (UTI) (8, 9). UTIs can mimic overactive bladder symptoms and can themselves worsen neurological symptoms (10).
The initial assessment should also include assessment of post micturition residual urine, before antimuscarinic medications are started. This measurement can be made by either using ultrasound or in-out catheterization. Urodynamic studies are not routinely performed unless LUT symptoms are refractory to treatment or intravesical treatment is being planned. Other urological/gynecological causes for LUT symptoms such as prostate enlargement or stress incontinence should be appropriately ruled out.
3.8.2.3 Management of LUT symptoms
The algorithm in Figure 3.8.1 outlines the management of LUT symptoms. Practical advice should be provided about cutting down caffeine, fizzy drinks and alcohol, as well as information about timed voiding and bladder retraining whenever appropriate. The fluid intake should be individualized, particularly taking into consideration possible concurrent cardiac issues; however, a fluid intake of between 1 to 2 liters a day is recommended (8). Pelvic floor exercises may be helpful especially when symptoms are mild.
Most individuals with FRDA and overactive bladder symptoms will require antimuscarinic medications (see Table 3.8.1 for a list of medications). The most often experienced side effects are dry mouth and constipation. If the former is too uncomfortable, then artificial saliva may be prescribed, in either tablet or spray form.
Antimuscarinic medications can increase heart rate, which may be relevant in a patient with cardiomyopathy. Cognitive problems can occur in FRDA (7), and in the rare situation where cognitive impairment is a feature, antimuscarinics should be prescribed with caution. In this setting, it would be sensible to use more selectively-acting medications such as trospium or darifenacin. If symptoms continue to be refractory, beta-3 receptor agonists such as mirabegron, percutaneous tibial nerve stimulation or intradetrusor injections of botulinum toxin A are options that could be considered.
Figure 3.8.1: Algorithm for managing LUT symptoms in Friedreich ataxia*
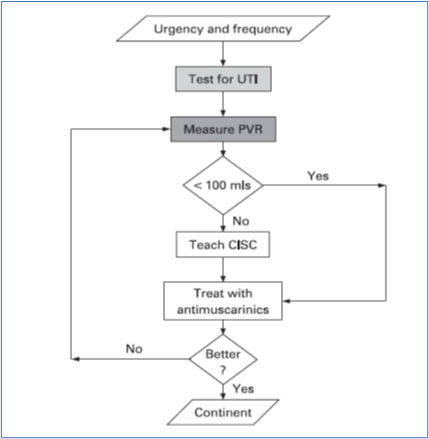
From the literature, voiding dysfunction and incomplete bladder emptying apparently occur uncommonly in people with FRDA. In individuals with persistently elevated post-void residual (PVR) volumes in excess of 100 mL, clean intermittent self-catheterization (CISC) is indicated. This should be taught by a urology specialist nurse or continence advisor. However, manual dexterity, truncal balance, cognitive function and vision would need to be considered when assessing suitability for CISC. With advancing disease, an indwelling catheter, preferably suprapubic, may be necessary.
Referral to specialist urology services is indicated in cases of hematuria; suspicion of a concomitant urological condition, e.g. prostatic enlargement; frequent urinary tract infections; and symptoms refractory to medical management. Referral should also be made when considering intradetrusor injections of botulinum toxin A or suprapubic catheter insertion.
Table 3.8.1: Antimuscarinic agents for managing overactive bladder symptoms

3.8.2.4 Management of recurrent urinary tract infection
Recurrent UTIs significantly impact the quality of life of individuals with neurological disorders and is one of the important causes for non-elective hospital admissions in patients with neurodegenerative disorders (11). There are no published studies evaluating the burden of recurrent UTIs in individuals with FRDA, or its management. In general, management of LUT dysfunction should be optimized and structural causes for UTIs, such as bladder stones or foreign bodies, should be excluded (12). The diagnostic evaluation includes ultrasonography of the urinary tract, urodynamic testing and cystoscopy. Other possible sources of infection should be considered, and bowel management and catheterization technique should be assessed and optimized if necessary.
Current literature does not support the routine use of antibiotic prophylaxis due to the lack of efficacy and increase in antimicrobial resistance. Recently, the concept of weekly oral cycling antibiotics appeared to show promising results in decreasing the incidence of UTI in spinal cord-injured patients without severe adverse events and colonization with multidrug-resistant bacteria (13), but further studies are warranted to see if these results can be replicated in the FRDA population. Several promising non-antibiotic options for UTI prophylaxis have also been developed, but further well-designed studies are similarly necessary to evaluate the safety and efficacy of these approaches in FRDA.
3.8.3 Overview of bowel function in Friedreich ataxia
3.8.3.1 Prevalence
Symptoms of bowel dysfunction are common in individuals with FRDA and a prevalence of 64% was reported in an observational study (1). Symptoms including constipation and fecal incontinence are also prevalent in the general population (14, 15).
3.8.3.2 Effects of Friedreich ataxia on bowel function
The spinal neurodegeneration of FRDA would be expected to result in gut symptomatology, in an analogous way to the symptoms that develop in patients with spinal cord injury (16). The etiology of these symptoms is complex; there may be autonomic and pelvic nerve dysfunction (with attenuation of voluntary motor function and impaired anorectal sensation and anorectal reflexes), or generalized systemic factors (e.g., altered diet and behavior, impaired mobility, psychological disturbances or drug adverse effects) (17).
3.8.4 Management of bowel dysfunction
The mainstay of current treatment is adopting a conservative approach and optimizing the mechanics of defecation through the use of laxatives and irrigation approaches (18). When successful, this approach improves both evacuation and incontinence symptoms, with associated improvements in quality of life and independence. Future therapies may be directed at modulating pelvic innervation through electrical stimulation.
Bowel symptoms in FRDA are frequently reported. Evaluation of bowel function should form part of the routine care of an individual with FRDA. Currently the etiology underlying reported bowel dysfunction is not clear. Studies aiming at understanding the pathophysiology of bowel symptoms in FRDA are urgently needed. The best practice statements and recommendation outlined here are based on experience and data from similar but distinct conditions. Studies evaluating the management of bowel dysfunction in people with FRDA would strengthen these recommendations.
Enquiring about lower urinary tract symptoms
QUESTION: Should enquiring about lower urinary tract (LUT) symptoms versus no assessment be used for all people with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: ↑LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We conditionally recommend that clinicians enquire about the presence of lower urinary tract (LUT) symptoms when consulting individuals with Friedreich ataxia.
JUSTIFICATION: Studies have shown that LUT symptoms are common in Friedreich ataxia (1) and clinical experience indicates that LUT symptoms may adversely affect quality of life in individuals with Friedreich ataxia and herald an increased risk of complications.
SUBGROUP CONSIDERATION: None.
Evidence to Recommendation Table PDFMeasuring post-void residual volume
QUESTION: Should measuring the post-void residual versus no assessment be used for all people with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: ↑LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We conditionally recommend that individuals with Friedreich ataxia reporting LUT symptoms have their post-void residual bladder volume measured.
JUSTIFICATION: Clinical experience indicates that LUT symptoms can significantly impact on quality of life. An elevated post-void residual (PVR) can exacerbate storage symptoms and also predispose to urinary tract infections. A PVR should be measured before commencing a trial of an antimuscarinic agent.
SUBGROUP CONSIDERATION: This recommendation is for individuals with Friedreich ataxia and LUT symptoms.
Evidence to Recommendation Table PDFAntimuscarinic agents for LUT symptoms
QUESTION: Should commencing antimuscarinic agents versus no treatment be used for patients reporting lower urinary tract (LUT) symptoms with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: ↑LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We conditionally recommend consideration of a trial of antimuscarinic agents in individuals with Friedreich ataxia with LUT symptoms reporting urinary storage symptoms.
JUSTIFICATION: Clinical experience indicates that LUT symptoms can significantly impact on quality of life and antimuscarinic agents can help to alleviate urinary storage symptoms.
SUBGROUP CONSIDERATION: This recommendation is for individuals with Friedreich ataxia with LUT symptoms reporting urinary storage symptoms. Measurement of post-void residual volume should be done before commencement of an antimuscarinic agent.
Evidence to Recommendation Table PDFIntermittent catheterization for urinary retention
QUESTION: Should intermittent catheterization versus indwelling catheter be used for people in urinary retention with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: ↑LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We conditionally recommend individuals with Friedreich ataxia in urinary retention undergo intermittent catheterization prior to insertion of an indwelling catheter, with suitability for catheterization dependent on their neurological abilities.
JUSTIFICATION: Clinical experience indicates that individuals with Friedreich ataxia can go into urinary retention and require intervention. However, in the case of recurrent or ongoing retention, intermittent catheterization may be difficult due to concurrent upper limb dysfunction. Furthermore, individuals with Friedreich ataxia may struggle with post-indwelling catheter trial of void due to neurogenic lower urinary tract dysfunction. These issues need to be considered on case by case basis in clinical management.
SUBGROUP CONSIDERATION: This recommendation is for individuals with Friedreich ataxia in urinary retention. An assessment of suitability for catheterization based on neurological abilities may be required.
Evidence to Recommendation Table PDFProphylactic antibiotics for recurrent urinary tract infection
QUESTION: Should prophylactic antibiotics versus not using prophylactic antibiotics be used for people presenting with recurrent UTI with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: ↑LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We suggest use of non-antibiotic agents, and if unsuccessful, antibiotic prophylactic agents over no antibiotic prophylaxis for the management of recurrent urinary tract infections (UTIs) in individuals with Friedreich ataxia.
JUSTIFICATION: There should be no fundamental difference in the treatment of recurrent UTIs in individuals with Friedreich ataxia as compared to individuals without Friedreich ataxia. Recurrent UTIs may lead to residual kidney damage and therefore should be managed. Due to potential for antibiotic resistance, non-antibiotic treatment could be tried first, but if unsuccessful, prophylactic antibiotics are warranted after discussion with expert urologists.
SUBGROUP CONSIDERATION: This recommendation is for individuals with Friedreich ataxia and recurrent UTIs. Individuals who are pregnant and presenting with recurrent UTIs should be referred to obstetrics for UTI management.
Evidence to Recommendation Table PDFEnquiring about bowel symptoms
QUESTION: Should enquiring about bowel symptoms versus none be used for all people with Friedreich ataxia?
STRENGTH OF RECOMMENDATION: ↑LEVEL OF EVIDENCE: ⨁◯◯◯
RECOMMENDATION: We conditionally recommend that clinicians enquire about the presence of bowel symptoms when consulting individuals with Friedreich ataxia.
JUSTIFICATION: Clinical experience indicates that bowel symptoms may adversely affect quality of life in individuals with Friedreich ataxia and herald an increased risk of complications, such as hemorrhoids, anal fissures, fecal impaction, bowel incontinence, rectal prolapse, bowel obstruction and urinary incontinence. Enquiring about bowel symptoms may lead to timely intervention to alleviate symptoms and avoid complications.
SUBGROUP CONSIDERATION: None.
Evidence to Recommendation Table PDFAntimuscarinic medications may be considered for people with Friedreich ataxia displaying overactive bladder symptoms.
Intradetrusor injections of botulinum toxin A or suprapubic catheterization may be considered as alternative intervention.
In a patient with persistently elevated post void residual volumes in excess of 100 mL, clean intermittent self-catheterization is indicated.
Consider modifying diet and lifestyle to optimize stool consistency and avoid fecal incontinence.
Titrate appropriate laxatives to optimize gut transit, stool consistency and avoid fecal impaction. Consider the use of prokinetic drugs.
Avoid fecal incontinence by treating fecal impaction if present. Facilitate prompt rectal evacuation via use of manual maneuvers, use of suppositories/mini enemas. Consider use of transanal irrigation and biofeedback behavioral therapy.
Lay summary of clinical recommendations for bladder and bowel problems in Friedreich ataxia
Why these recommendations?
Studies show that people with Friedreich ataxia may have changes in bladder or bowel function.
Bladder problems can include: difficulties with holding urine due to urinary urgency; more visits to the toilet in the daytime; waking up at night with the need to pass urine (known as nocturia); and leaking of urine (known as incontinence) when unable to get to the toilet in time. These are known as overactive bladder symptoms. Individuals can also have problems when passing urine (called voiding), such as: difficulty starting the process of urination; an interrupted urine stream; feeling a sensation of incomplete bladder emptying after urinating; passing urine more than once when you visit the toilet (called double voiding); and sometimes retaining urine in the bladder afterwards.
Bowel symptoms can include constipation, bowel urgency and fecal incontinence.
These symptoms often have a major impact on the quality of life of an individual with Friedreich ataxia, and frequently have an impact on the carers.
We therefore recommend that healthcare professionals should ask people with Friedreich ataxia whether they have bladder or bowel symptoms. It is beneficial for patients to talk to their neurologist and other healthcare professionals about any bladder and bowel symptoms they may be experiencing, as these symptoms are an important aspect of Friedreich ataxia.
In terms of treatment, there is currently little research evidence for the effects of treatments for bladder and bowel problems specifically in people with Friedreich ataxia. However, treatments used for individuals with other neurological disorders can be tried.
For problems with bladder control:
• In the first instance, measures such as a review of fluid intake and undertaking pelvic floor exercises should be tried, particularly when symptoms are mild. Dietary changes such as cutting back on coffee, tea, orange juice, fizzy sugary drinks and artificial sweeteners, and reducing alcohol intake may help.
• Before starting medications, urinary tract infections (UTIs) should be excluded by testing the urine and measuring the volume of urine remaining in the bladder after voiding (known as the post-void residual volume).
• If UTI has been excluded and symptoms persist, individuals should be offered medications that calm the overactive bladder. These medications have been found to be helpful in clinical practice, although no controlled research trials have been performed in people with Friedreich ataxia.
• If these medications are not effective then your doctor may consider trying an alternative treatment, such as an injection of botulinum toxin into the bladder wall.
For bowel symptoms:
• Changing your diet and lifestyle may help improve the consistency of stools: dedicating time to meals and bowel function is an important part of lifestyle change.
• Different types of laxatives are available for managing constipation.
• Fecal incontinence may be caused by hard stools that are difficult to pass (fecal impaction), so it is important to avoid this situation through diet and lifestyle. Bowel incontinence may also relate to very loose stools from inappropriate use of laxatives.
• Trans-anal irrigation (enema/clyster) may be considered for optimal emptying of the rectum.
What does this mean for you as a person living with Friedreich ataxia or caring for someone living with Friedreich ataxia?
It is important for you to speak with your healthcare professional about Friedreich ataxia and your bladder and bowel function, what it means for you, and what treatments may be useful (depending on your symptoms).
Who are these recommendations specifically for?
These recommendations are specifically for individuals with Friedreich ataxia who report bladder and/or bowel symptoms.
Consultant NeuroGastroenterologist, National Hospital Neurology & Neurosurgery, London, UK
Paola Giunti, MD, PhD
Professor, Queen Square Institute of Neurology, UCL, London, UK
Email: p.giunti@ucl.ac.uk
Marios Hadjivassiliou, MD
Professor of Neurology, Sheffield Teaching Hospitals NHS Trust and University of Sheffield, Sheffield, South Yorkshire, UK
Jalesh N. Panicker, MD, DM, FRCP
Associate Professor and Consultant Neurologist in Uro-Neurology, The National Hospital for Neurology and Neurosurgery and UCL Queen Square Institute of Neurology, London, UK
David J. Szmulewicz, MBBS, PhD, FRACP
Neurologist, The Florey Institute of Neuroscience and Mental Health, Melbourne, Victoria, Australia
Email: dsz@me.com
1. Lad M, Parkinson MH, Rai M, Pandolfo M, Bogdanova-Mihaylova P, Walsh RA, et al. Urinary, bowel and sexual symptoms in a cohort of patients with Friedreich’s ataxia. Orphanet J Rare Dis. 2017;12(1):158.
2. Musegante A, Almeda P, Monteiro R, Bassoro U. Urinary symptoms and urodynamics findings in patients with Friedreich’s ataxia. International Brazilian Journal of Urology. 2013;39(6):867-74.
3. Delatycki MB, Paris DB, Gardner RJ, Nicholson GA, Nassif N, Storey E, et al. Clinical and genetic study of Friedreich ataxia in an Australian population. Am J Med Genet. 1999;87(2):168-74.
4. Dürr A, Cossee M, Agid Y, Campuzano V, Mignard C, Penet C, et al. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med. 1996;335(16):1169-75.
5. Vezina JG, Bouchard JP, Bouchard R. Urodynamic evaluation of patients with hereditary ataxias. Can J Neurol Sci. 1982;9(2):127-9.
6. Nardulli R, Monitillo V, Losavio E, Fiore P, Nicolardi G, Megna G. Urodynamic evaluation of 12 ataxic subjects: neurophysiopathologic considerations. Funct Neurol. 1992;7(3):223-5.
7. Schulz JB, Boesch S, Burk K, Durr A, Giunti P, Mariotti C, et al. Diagnosis and treatment of Friedreich ataxia: a European perspective. Nat Rev Neurol. 2009;5(4):222-34.
8. Fowler CJ, Panicker JN, Drake M, Harris C, Harrison SC, Kirby M, et al. A UK consensus on the management of the bladder in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2009;80(5):470-7.
9. Coyne KS, Kaplan SA, Chapple CR, Sexton CC, Kopp ZS, Bush EN, et al. Risk factors and comorbid conditions associated with lower urinary tract symptoms: EpiLUTS. BJU Int. 2009;103 Suppl 3:24-32.
10. Hufschmidt A, Shabarin V, Rauer S, Zimmer T. Neurological symptoms accompanying urinary tract infections. Eur Neurol. 2010;63(3):180-3.
11. Low V, Ben-Shlomo Y, Coward E, Fletcher S, Walker R, Clarke CE. Measuring the burden and mortality of hospitalisation in Parkinson’s disease: A cross-sectional analysis of the English Hospital Episodes Statistics database 2009-2013. Parkinsonism Relat Disord. 2015;21(5):449-54.
12. Pannek J, Wollner J. Management of urinary tract infections in patients with neurogenic bladder: challenges and solutions. Res Rep Urol. 2017;9:121-7.
13. Salomon J, Denys P, Merle C, Chartier-Kastler E, Perronne C, Gaillard JL, et al. Prevention of urinary tract infection in spinal cord-injured patients: safety and efficacy of a weekly oral cyclic antibiotic (WOCA) programme with a 2 year follow-up–an observational prospective study. J Antimicrob Chemother. 2006;57(4):784-8.
14. Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750-9.
15. Perry S, Shaw C, McGrother C, Matthews RJ, Assassa RP, Dallosso H, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002;50(4):480-4.
16. Chung EA, Emmanuel AV. Gastrointestinal symptoms related to autonomic dysfunction following spinal cord injury. Prog Brain Res. 2006;152:317-33.
17. Craggs MD, Balasubramaniam AV, Chung EA, Emmanuel AV. Aberrant reflexes and function of the pelvic organs following spinal cord injury in man. Auton Neurosci. 2006;126-127:355-70.
18. Preziosi G, Emmanuel A. Neurogenic bowel dysfunction: pathophysiology, clinical manifestations and treatment. Expert Rev Gastroenterol Hepatol. 2009;3(4):417-23.
These Guidelines are systematically developed evidence statements incorporating data from a comprehensive literature review of the most recent studies available (up to the Guidelines submission date) and reviewed according to the Grading of Recommendations, Assessment Development and Evaluations (GRADE) framework © The Grade Working Group.
This chapter of the Clinical Management Guidelines for Friedreich Ataxia and the recommendations and best practice statements contained herein were endorsed by the authors and the Friedreich Ataxia Guidelines Panel in 2022.
It is our expectation that going forward individual topics can be updated in real-time in response to new evidence versus a re-evaluation and update of all topics simultaneously.

